Ensuring Every Batch is a Success: Validation and Qualification in Cell and Gene Therapy Manufacturing
Have you ever followed a recipe step by step to end up with something less than delicious and not what you were after? In the world of pharmaceutical manufacturing, including the manufacture of cell and gene therapy products, the stakes are much higher than a burnt cake or a bad batch of fried rice. Here, ensuring every “recipe” produces safe and effective medicines requires a rigorous process called validation and qualification.
Validation is a systematic approach that involves establishing documented evidence through a series of processes to confirm that a particular manufacturing process will consistently produce products that meet predefined quality standards. Simple, right? Let’s break it down to relatable levels.
Imagine a fancy kitchen with top-of-the-line equipment: sleek ovens, blenders, and a pressure cooker that promises perfect rice every time. Qualification is like testing this equipment. We check if the oven maintains temperature, the blender chops evenly, and the pressure cooker performs as advertised. This ensures they’re fit for their assigned jobs.
However, even with perfect equipment, a perfectly cooked dish isn’t guaranteed. We still need to follow the recipe, and the process, by adding the right ingredients, measuring accurately, and cooking for the correct time. Validation is like testing this recipe. We make repeated batches of rice, measuring every step and analysing the final product. This confirms that the entire process consistently produces perfectly cooked rice- not just occasionally. Validation ensures accuracy.
This is a simplified analogy, and the actual validation and qualification processes in pharmaceutical manufacturing involve complex scientific testing and data analysis.
So how do we translate this culinary analogy to apply to within the cell and gene therapy landscape?
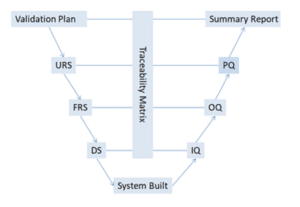
At Cell Therapies, we follow what is called a ‘V-model of validation’ that ensures both the individual pieces of equipment (left side of the V) and the entire manufacturing process (right side of the V) work together seamlessly to produce safe and effective medications.
The Qualification and Validation Processes
When checking the quality of our cell and gene therapy equipment, facility, and utilities – there are a range of questions that must be asked for each item.
For example, do packaging machines consistently produce accurate and tamper-proof containers? Are the mixing tanks properly cleaned and sterilized? Does the quality control analytical equipment have any defects? Can products freeze at a controlled temperature rate that’s specified by the end-user over the entire process?
All of these are just some of the questions that must be asked during the qualification process to ensure each item is working the way it is supposed to ensure accuracy before the validation process. If there are no qualification processes before validation, the product is likely to face difficulties that could have been avoided.
Similar thinking occurs during the validation process, except this time it’s not the equipment being checked but rather the accuracy of the manufacturing process. There are once again numerous questions that the Quality Control team must ask and check to ensure that cell-based therapy will go as desired.
For example, are the correct amounts of active ingredients and other components precisely measured every time? Do the steps follow the recipe exactly to ensure the desired cell types are expressed? Are rigorous checks performed at each stage to confirm quality and consistency?
The Importance
Validation and qualification aren’t just fancy buzzwords; they’re crucial for patient safety and product quality. They minimize the risk of errors, ensure medications perform as intended, and meet strict regulatory requirements.
The next time you take a medication, remember the rigorous testing and meticulous processes that went into ensuring its safety and effectiveness. A team of quality and validation experts is essentially like having a team of expert chefs meticulously testing a recipe, the raw materials and the equipment used in the recipe – except with even higher stakes and more meaningful results – saving lives.
Blog is written by Harry Wan in conjunction with the Cell Therapies Marketing department.