Cell Therapies provides integrated, phase-appropriate development and GMP manufacturing of cell and gene therapies under one roof, from preclinical concept to commercial supply, minimizing transition risk and preserving critical program knowledge across the cell therapy manufacturing lifecycle. We specialize in the manufacture of cell therapies, ex-vivo gene therapies, and regenerative medicine products.
Australian-based CDMO for Cell and Gene Therapy
Where Science Scales, and Clinical and Commercial Manufacturing Converge

Cell Therapies in numbers
In the last four years we delivered:
142
99
10
6
4
Asia Pacific’s Leading End-to-end Cell and Gene Therapy Manufacturing Partner
Our partners and clients
Our Services
Manufacturing life changing cell and gene therapies for over 20 years
Cell Therapies evolved from academic roots to become a globally recognized CDMO with commercial manufacturing expertise. We offer clinical-grade, regulatory-compliant GMP manufacturing while maintaining translational agility. Our end-to-end model balances cost-effectiveness, scalability, and scientific depth, supporting programs throughout development, from first-in-human clinical trials through to commercial supply.
GMP Manufacturing from First-in-Human Clinical Trials to Commercial Supply
Our experience in GMP manufacturing of complex and sophisticated cell-based therapies with global reach can support your program. We offer licensed GMP manufacturing for autologous and allogeneic cell therapy products throughout the development pipeline, from translational first-in-human clinical studies to late-phase trials and commercial supply.
Our two decades of experience span autologous and allogeneic therapies across a wide range of cell types, including chimeric antigen receptor (CAR) T-cells, mesenchymal stromal cells, tumour-infiltrating lymphocytes (TILs), and human pluripotent stem cells and their derivatives.
Seamless and Scalable Program Onboarding
Our technology transfer framework enables efficient onboarding of complex cell therapy programs into our GMP facility. We apply a phase-appropriate, risk-based approach across six gated stages from technical feasibility assessment, knowledge capture, to process implementation, ensuring operational continuity and reducing transition risk.
With over ten (10) successful technology transfers in the last 4 years (including commercial CAR-T programs), our team is adept at scaling diverse modalities under strict regulatory conditions.
End-to-End Cold Chain Management
Cell Therapies provides validated cryopreservation of clinical materials, including apheresis collections, in-process intermediates, and final products, using vapor-phase liquid nitrogen systems. Our GMP-compliant workflows include labeling, storage, and full-chain-of-custody documentation ensuring alignment with regulatory requirements for both clinical and commercial programs.
Our logistics partnerships and in-house support enable reliable, time-critical delivery across Australia, the Asia-Pacific region, and global clinical trial networks.
Our facility houses validated vapour-phase liquid nitrogen cryostorage and integrated digital inventory systems. With established logistics partners, we support qualified cryo-transport to hospitals and clinical sites across Australia, Asia-Pacific, and globally. We also support direct-to-patient delivery models for time-sensitive autologous therapies.
From day-zero patient apheresis to final filled vials and bags, Cell Therapies safeguards cell viability with industry‑leading cryo & cold‑chain protocols.
Enabling Translation and Commercial Readiness
Innovation meets manufacturability in our purpose‑built Process Development Laboratory. Our Process Development capabilities include expertise in closed systems, automation, and rapid process scale-up implementation for early-stage clinical products; while focusing on cost reduction, standardization, and process robustness for late-stage clinical and commercial products. We utilize a dedicated PC2/BSL2 development laboratory with access to emerging platform technologies and equipment to drive translation to the clinic.
Cell Therapies’ deep expertise and experience in manufacturing of complex cell-based products supports translation of pre-clinical research through the clinic to the market.
With over two decades of experience in GMP manufacturing of cell-based products, Cell Therapies is a trusted partner in transforming promising research into scalable, compliant, and commercially viable therapies.
Our dedicated Development Laboratory offers clients early access to established and emerging platforms, enabling the design of robust, phase-appropriate manufacturing processes grounded in real-world GMP requirements.
Ensuring safety and efficacy through cutting-edge analytics
Assays that satisfy regulators and investors alike – Cell Therapies’ Quality Control (QC) laboratories combine gold-standard methods with novel analytics and digital traceability to de-risk release and stability profiles across autologous and allogeneic cell therapies. With deep regulatory experience across the clinical product lifecycle, we support early- and late-stage programs with robust assay development, validation, and compliance infrastructure.
Our in-house analytical and QC capabilities include:
- TGA-licensed flow cytometry (21 CFR Part 11 compliant)
- ddPCR and qPCR-based assays
- ELISA and cell-based potency assays
- Rapid sterility via BacT/ALERT
- Endotoxin and mycoplasma testing
- Stability testing with cryopreservation protocols
Strategic Guidance to De-Risk Development and Accelerate Timelines
Cell Therapies’ consulting arm is powered by scientific, regulatory, and operational leaders who’ve guided dozens of cell and gene therapy programs globally. From concept to IND to market, Cell Therapies supports your entire advanced therapy journey.
Cell Therapies’ consulting and advisory capability is a vital service that can assist clients to advance their cell and gene therapy program from pre-clinical development to treating clinical patients and future commercialisation.
We have expertise in navigating the challenges of manufacturing processes, regulatory requirements, quality standards, clinical needs, and logistics for the delivery of cell and gene therapies to patients in Australia and Asia Pacific region.
Services Offered
- CMC Road-mapping & Regulatory Gap Assessments
- FACT Accreditation & GMP Readiness Support
- Quality Management Consulting (quality by design (QbD), risk assessments)
- Facility Build-vs-Buy Feasibility For Early-stage Innovators
- Asia-Pacific Clinical Feasibility Assessment and Country Selection
- Regulatory Agency Meeting Support and Submission Strategy
- Quality Consultation as a Service (QCaaS) (ongoing subject matter expert (SME) access
Inspection-Ready Quality and Quality Management Systems Tailored to Your Program
Cell Therapies’ quality systems are globally recognized and inspection-ready, having passed reviews by TGA, FDA, PMDA, and EMA. We offer flexible, fit-for-purpose Quality Management System (QMS) solutions for biotech companies, hospitals, and academic groups. Services include gap assessments, audit preparation, regulatory compliance support, validation support, and ongoing QA/QC consultation.
For clients without in-house quality infrastructure, we act as an embedded partner, ensuring your program remains compliant, efficient, and always inspection-ready. We also provide interim quality leadership as a service.
Integrated Trial Supply Across Australia and the Asia-Pacific
Embedded within the Peter MacCallum Cancer Centre, one of the largest cancer research hospitals in the Southern Hemisphere, we are uniquely positioned to support clinical trial supply in Australia and across APAC. Our integration with hospital networks and clinicians ensures that advanced therapies are delivered safely, efficiently, and in compliance with regulatory requirements.
Cell Therapies provides GMP manufacturing, cryopreservation, and validated logistics to enable smooth trial execution. With regulatory alignment through the TGA and recognition by global agencies, we help sponsors accelerate trial initiation and patient access while minimizing operational risk.
Our State-of-the-art Facility
As Australia’s only biomedical manufacturing facility where CAR T-cells and other “living therapies” can be made at a commercial scale, Cell Therapies support high throughput manufacture of clinical trial and commercial cell and gene therapy products for Australian patients and the Asia-Pacific region.
How We Work
Our gated six step process to work with Cell Therapies
From Initial engagement to manufacturing your first clinical batch, what does the process look like? While complex and technical, we have simplified it down to these 6 simple steps:







Our Partners, Memberships and Associations
Ecosystem Engagement To Drive Sector Growth
Cell Therapies supports the growth of the Australian and Asia-Pacific cell and gene therapy sector through partnerships and collaborations with biotech companies, pharmaceutical companies, healthcare, industry, and government.
Our commitment to GMP manufacturing excellence of complex cell-based therapies underpins our Purpose and Values.
News and Resources
Find out the latest Cell Therapies news and insights from our in-house experts.
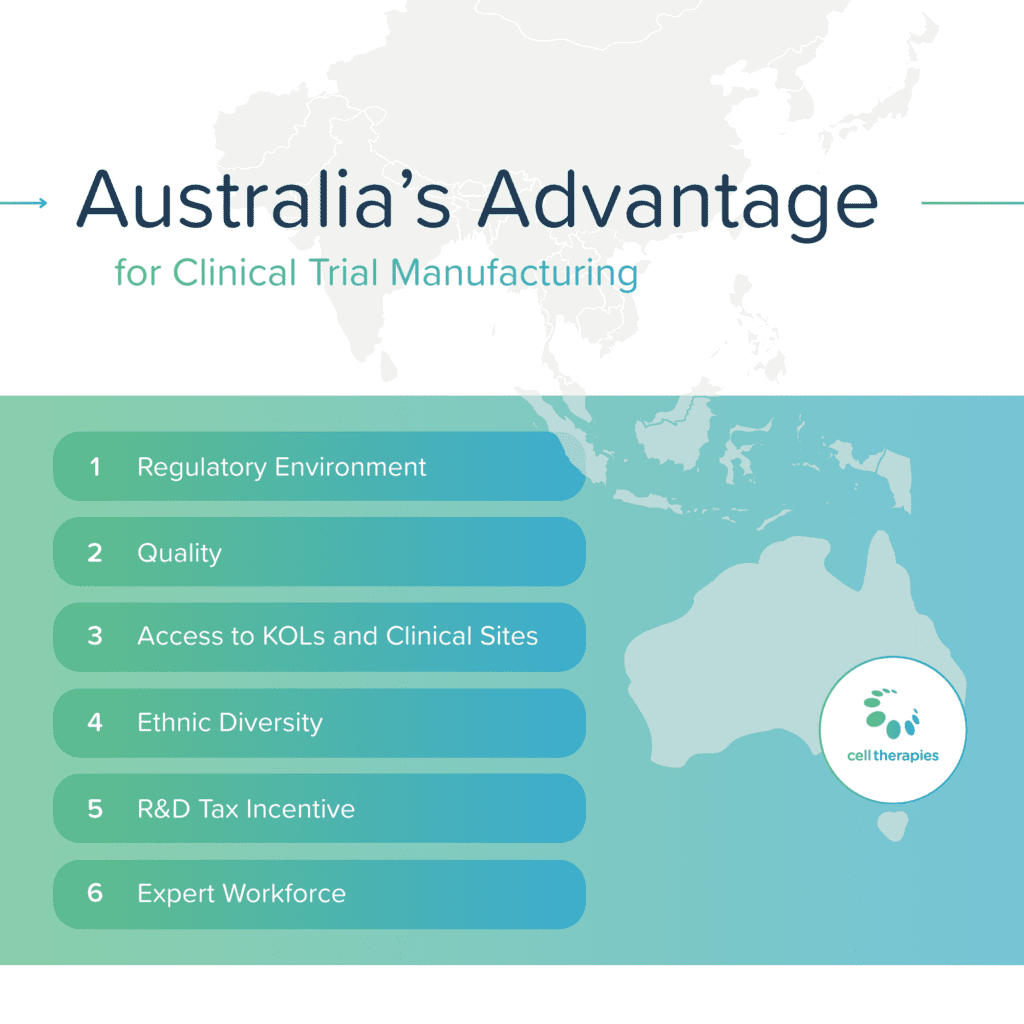
Phase-appropriate GMP and Regulatory Support
True Quality Comes at a Cost — and It’s Worth It
Cell Therapies is your strategic partner across the entire development journey. From early translation and first-in-human clinical trials, to commercial supply, our state-of-the-art facility and expert teams enable cell and gene therapy programs to thrive in the Asia-Pacific region.
Quality is not a box to tick. It’s our backbone. our Quality Management System (QMS) has passed inspections from TGA, FDA, EMA, PMDA, MFDS, and HSA. We don’t compromise quality.


Working at Cell Therapies
Establish and Advance Your Cell and Gene Therapy Career
Cell Therapies encourages an environment in which everyone can be their true selves and belong. We are driven by our Purpose to deliver the promise of advanced therapies.
Equality, diversity and inclusion are important facets of our company. Our Values of connection, respect, curiosity and integrity guide our interactions with co-workers and clients every day.